Using Mass Describe the Relative Size of the Subatomic Particles
As a result an atom consists largely of empty space. The Importance of these three subatomic particles are given below.

Phosphorus Atomic Structure Google Search Atomic Structure Sodium General Physics
Using mass describe the relative size of the subatomic particles.
. The subatomic particles of atoms are Protons Neutrons. A composite particle proton is made of two up quark and one down quark which are elementary particles. Answer 1 of 5.
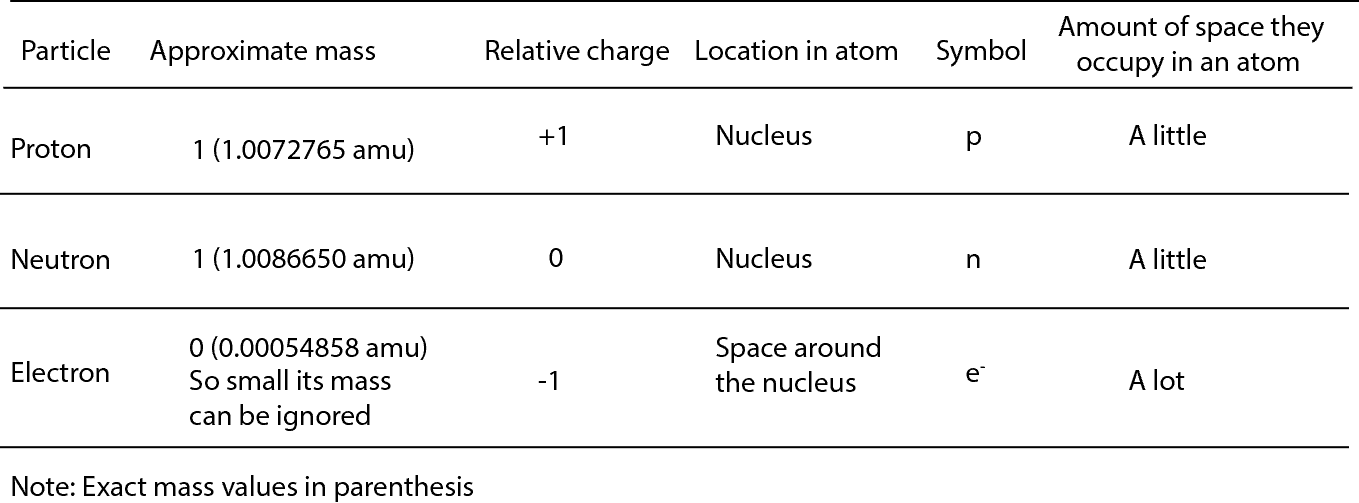
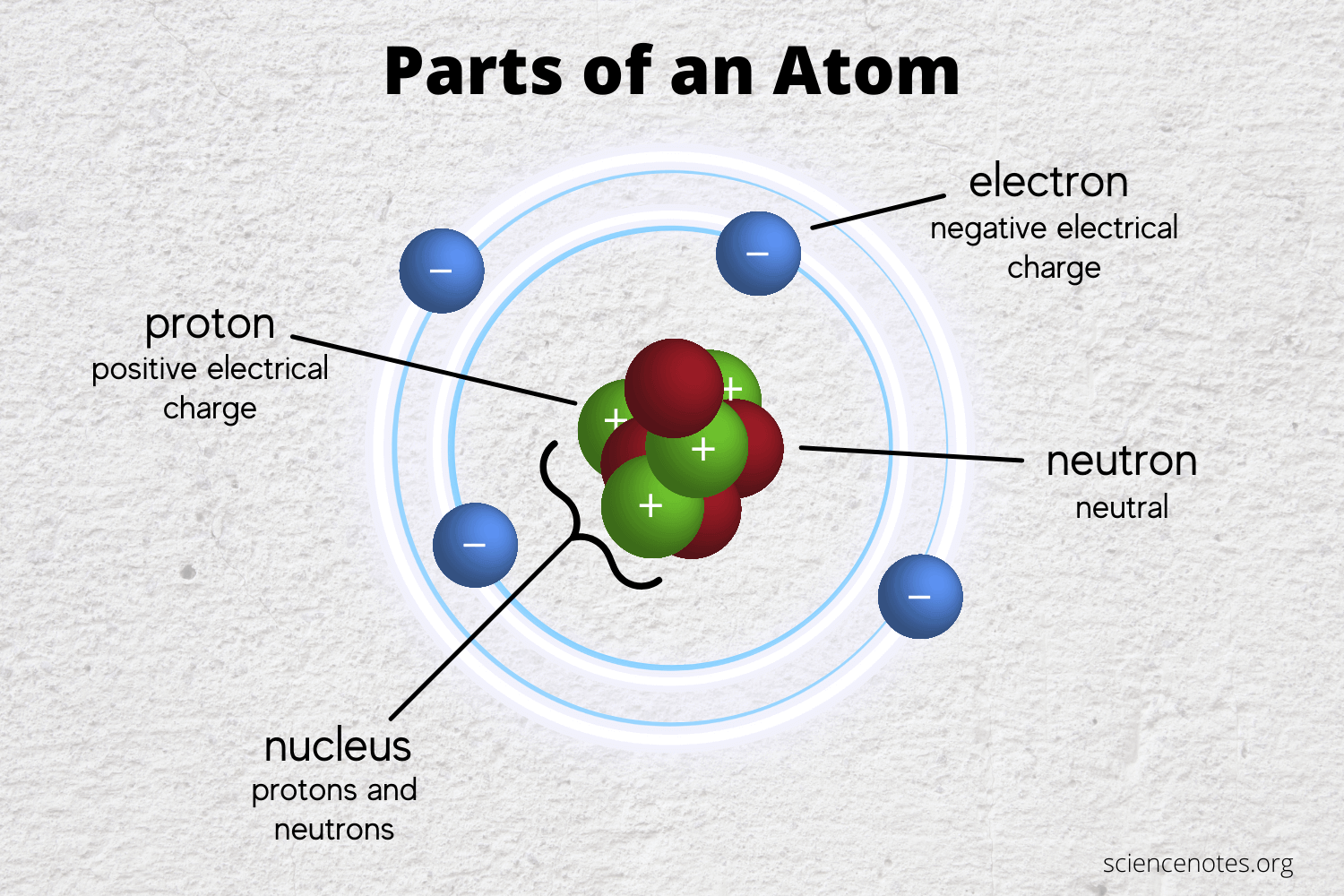
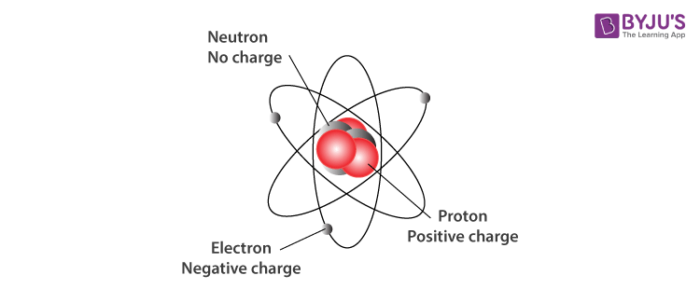
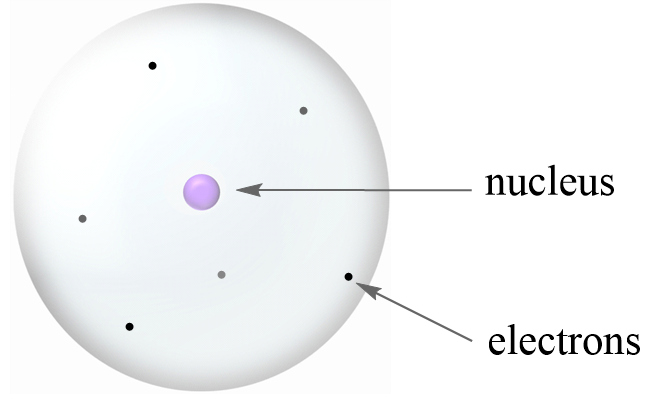
The neutrons are located inside the nucleus and have no charge. Proton charge of e in the nucleus Neutron 0 charge in the nucleus and Electron charge of e outside the nucleus. In nature an important rule is that positive charged particles always attract negative charged particles and negative charged particles always repel.
Actually I found several comparisons that all seem a little different. The properties of these particles are summarized in the table below. The masses of subatomic particles are very tiny.
Then tell students that the distance of the electron from the. What is the rationale for using the relative masses and the relative charges of subatomic particles rather than their absolute masses and charges. The rest masses are slightly different which is explained below.
Most of the mass of an atom is in the nucleus while the orbiting electrons account for an atoms size. A proton has a mass of 16726 x 10-24grams. Describe the three main subatomic particles.
If I tell you that the lithium nucleus has a charge of 3 you know it has 3 protons. Protons electrons and neutrons. And they are related to one another by their mass and charge.
The relative mass of a proton is 1 and a particle with a relative mass smaller than 1 has less mass. Subatomic particle Relative mass Relative charge Proton 1 1 Neutron 1 0 Electron 00005 -1. A proton has a mass approximately 1836 times greater than the mass of the electron but the masses of protons and neutrons differ less than one percent.
Relative Mass proton 1 Relative Charge. The protons are located inside the nucleus and have a positive charge. The building blocks of elements Proton.
The main three subatomic particles are Protons electrons and neutrons. The electrons are located outside the nucleus and have a negative charge. Protons including neutrons give the atom mass but are not involved in chemical reactions.
The mass of electrons is very small compared to protons and neutrons. The SI unit for mass is the kilogram - whether for an atomic particle of for a galaxy. Instead of writing their actual masses in kilograms we often use their relative masses.
K0 has a PDG rest mass value of 04976 GeV. The relative charge of protons is 1 the relative mass of a proton is 1 and a proton is located in the center of the atomnucleus. Of subatomic particles is measured in atomic mass units amu.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. - illustrate the structure of the atom by using the Bohr model including the charge relative mass and location of the sub-atomic particles. Imagine the atom as the size of a professional baseball stadium in 3D.
Positive charge found in nucleus 1008 amu. The proton has a mass of 1007 amu the neutron has a mass of 1 amu and the electron has a mass of 0005 amu. This particle has a charge of zero.
The size of the nucleus would be about the size of a baseball in proportion. Protons Protons are particles that are positively charged and are also located in the nucleus. The mass of a proton is about 1800 times greater than the mass of an electron.
Atom is defined as the smallest unit of a matter. This is a positively charged particle that is present in the nucleus of atoms. List the subatomic particles and describe their relative masses charges and positions in the atom Atom.
It has a charge of 16 10-19 C. The protons and neutrons are found in the nucleus at the centre of the atom. But for ease we might say it has a charge of e or 1.
Two are charged particles K-and K and one is a neutral particle K0. Atomic Structure - describe the characteristics of protons neutrons and electrons in terms of location charge and mass. E3radg8 and 1 more users found this answer helpful.
The subatomic particles are. Ants would be far too big to represent as the electrons. What is the Lightest.
That an atom is 999999 empty space. Atoms contain three sub-atomic particles called protons neutrons. Atom is made up of three subatomic particles.
K-and K have a PDG rest mass value of 04937 GeV. - analyze the structure of the. - use atomic mass atomic number and charge to identify neutral atoms ions and isotopes.
Protons neutrons and electrons. Properties of Subatomic Particles Particle Charge Relative mass Proton 160 x 10-19 C 1 amu Electron -160 x 10-19 C 0 amu 11840 amu Neutron neutral 1 amu. Considering all the information about atomic size.
In physical sciences a subatomic particle is a particle that is smaller than an atom. Provide students with information regarding the relative mass of the proton and the electron. 1675 10 27.
There are three kaons K. Jump to navigation Jump to search. What is a unit to measure mass of a subatomic particles.
What are the location charge and mass of the three subatomic particles. Protons are practically the same size as neutrons and both are much larger than electrons. Like other charged particles the difference in rest mass value fits within.
P 1673 10 27. The relative charge of the neutron is 0 the relative mass of a neutron is 1 and the neutron is located in the center of atomsnucleus along with protons. Particle whose size or mass is less than that of the atom.

Matter Atoms Quarks Leptons Fizik Ve Matematik Kuantum Mekanigi Fizik Bilimi
The Famous Types Of Subatomic Particles Praxilabs

What Is Antimatter Weak Interaction Electromagnet Quantum Mechanics

Atomic Structure And Subatomic Particles Youtube

Tetryonics 75 04 Doppler Shifted Wavelengths Of Energy Momentum Wavelength Or Frequency Are The Result Of Time Based Quantum World Quantum Leap Math Methods

Unit 3 Atomic Structure Flashcards Quizlet

The Mass And Size Of The Subatomic Particles Protons Neutrons And Electrons Youtube

Atomic Structure Nucleus Contains Protons And Neutrons Matter Science Relative Atomic Mass Atomic Structure

Results For Pbs Learningmedia Resource Classroom Pbs Learning Media Science Classroom

Pin By Girl Scouts North Carolina C On Stem Stem Camp Stem Careers Stem Girls
What Size Are The Particles Of An Atom In Relation To Its Size Quora

Iv Isotopes 2 Or More Atoms Of The Same Element Having The Same Number Of Protons But Different Numbers Of Neutrons Atomic Theory Neutrons Atom

Fantastic Back To School Review Game This Interactive Game Reviews Students The Concept Of The Periodic Table Atoms Elements And The Perio Atoms Pinte
Subatomic Particles Definition Discovery And Key Features

What Re The Properties Of The Subatomic Particles And How Are These Particles Related

Normal Force Card Sort Concept Builder Is Based On A Popular Card Sorting Idea In Which Students Are Given A Collection Of Car Normal Force Sorting Cards Force

Pin By Sara Al Ahmade On Chemistry Electron Configuration Electron Affinity Ionization Energy
Comments
Post a Comment